Puzzling New Behavior Found in High-Temperature Superconductors
Research led by SLAC and Stanford scientists has uncovered a new, unpredicted behavior in a copper oxide material that conducts electricity without any loss at relatively high temperatures.
Research by an international team led by SLAC and Stanford scientists has uncovered a new, unpredicted behavior in a copper oxide material that becomes superconducting – conducting electricity without any loss – at relatively high temperatures.
This new phenomenon – an unforeseen collective motion of electric charges coursing through the material – presents a challenge to scientists seeking to understand its origin and connection with high-temperature superconductivity. Their ultimate goal is to design a superconducting material that works at room temperature.
"Making a room-temperature superconductor would save the world enormous amounts of energy," said Thomas Devereaux, leader of the research team and director of the Stanford Institute for Materials and Energy Sciences (SIMES), which is jointly run with SLAC. "But to do that we must understand what's happening inside the materials as they become superconducting. This result adds a new piece to this long-standing puzzle.”
The results are published Oct. 19 in Nature Physics.
Delving Into Doping Differences
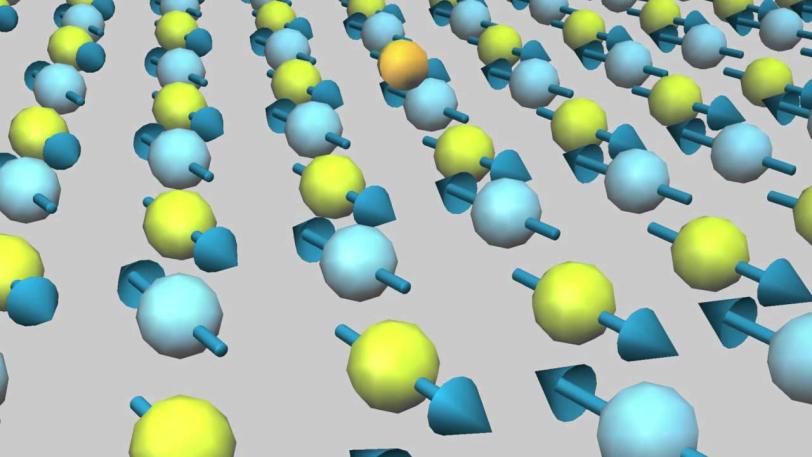
The researchers used an emerging X-ray technique called resonant inelastic X-ray scattering, or RIXS, to measure how the properties of a copper oxide change as extra electrons are added in a process known as doping. The team used the Swiss Light Source’s RIXS instrument, which currently has the world's highest resolution and can reveal atomic-scale excitations – rapid changes in magnetism, electrical charge and other properties – as they move through the material.
Copper oxide, a ceramic that normally doesn’t conduct electricity at all, becomes superconducting only when doped with other elements to add or remove electrons and chilled to low temperatures. Intriguingly, the electron-rich version loses its superconductivity when warmed to about 30 degrees above absolute zero (30 kelvins) while the electron-poor one remains superconducting up to 120 kelvins (minus 244 degrees Fahrenheit). One of the goals of the new research is to understand why they behave so differently.
The experiments revealed a surprising increase of magnetic energy and the emergence of a new collective excitation in the electron-rich compounds, said Wei-sheng Lee, a SLAC staff scientist and lead author on the Nature Physics paper. “It’s very puzzling that these new electronic phenomena are not seen in the electron-poor material,” he said.
Lee added that it’s unclear whether the new collective excitation is related to the ability of electrons to pair up and effortlessly conduct electricity – the hallmark of superconductivity – or whether it promotes or limits high-temperature superconductivity. Further insight can be provided by additional experiments using next-generation RIXS instruments that will become available in a few years at synchrotron light sources worldwide.
A Long, Tortuous Path
This discovery is the latest step in the long and tortuous path toward understanding high-temperature superconductivity.
Scientists have known since the late 1950s why certain metals and simple alloys become superconducting when chilled within a few degrees of absolute zero: Their electrons pair up and ride waves of atomic vibrations that act like a virtual glue to hold the pairs together. Above a certain temperature, however, the glue fails as thermal vibrations increase, the electron pairs split up and superconductivity disappears.
Starting in 1986, researchers discovered a number of materials that are superconducting at higher temperatures. By understanding and optimizing how these materials work, they hope to develop superconductors that work at room temperature and above.
Until recently, the most likely glue holding superconducting electron pairs together at higher temperatures seemed to be strong magnetic excitations created by interactions between electron spins. But a recent theoretical simulation by SLAC and Stanford researchers concluded that these high-energy magnetic interactions are not the sole factor in copper oxide’s high-temperature superconductivity. The new results confirm that prediction, and also complement a 2012 report on the behavior of electron-poor copper oxides by a team that included Lee, Devereaux and several other SLAC/Stanford scientists.
“Theorists must now incorporate this new ingredient into their explanations of how high-temperature superconductivity works,” said Thorsten Schmitt, leader of the RIXS team at the Paul Scherrer Institute in Switzerland, who collaborated on the study.
Other researchers involved in the study were from Columbia University, University of Minnesota, AGH University of Science and Technology in Poland, National Synchrotron Radiation Research Center and National Tsing Hua University in Taiwan, and the Chinese Academy of Sciences. Funding for the research came from the DOE Office of Science, U.S. National Science Foundation and Swiss National Science Foundation.
Citation: W. S. Lee, et al., Nature Physics, 19 October 2014 (10.1038/nphys3117)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
The Stanford Institute for Materials and Energy Sciences (SIMES) is a joint institute of SLAC National Accelerator Laboratory and Stanford University. SIMES studies the nature, properties and synthesis of complex and novel materials in the effort to create clean, renewable energy technologies. For more information, please visit simes.slac.stanford.edu.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.